This is breaking news. The UK aesthetics industry has experienced a meteoric rise in the last two decades. It has ballooned into a £3.6 billion market. Its rapid expansion has been fueled by consumer demand for non-surgical procedures such as Botox, dermal fillers and the increasingly popular body contouring treatments such as the Brazilian Butt Lift (BBL).
While this growth reflects societal shifts towards body positivity and self-care, it has also spotlighted significant safety and regulatory gaps within the sector. The UK government, alongside professional bodies and advocacy groups, is now addressing these concerns through a series of reforms.
A Growing Industry with Growing Pains
The aesthetics sector has an appeal that lies in the promise of minimally invasive treatments that deliver dramatic results with shorter recovery times compared to traditional cosmetic surgery. This is the real power of the sector. However, industry success has come at a cost. A surge of unqualified practitioners and the lack of enforceable standards have left many patients vulnerable to botched procedures, life-altering injuries and more recently, even fatalities.
In response, the UK government has proposed a mandatory licensing scheme aimed at improving safety and professionalism across the sector. Empowered by an amendment to the Health and Care Act in 2022, the Secretary of State for Health and Social Care now has the authority to introduce new regulations. Ensuring that only qualified practitioners perform aesthetic interventions. The proposed scheme could criminalise unlicensed practice, bringing a much-needed layer of accountability to the industry.
The government’s initiative has involved extensive public consultation, seeking input from practitioners, patients, and stakeholders. Key questions include which treatments should fall under the licensing scheme, the minimum qualifications required, and whether high-risk procedures should only be performed by healthcare professionals regulated by the Care Quality Commission (CQC).
Charlotte Steele, a spokesperson for the Department of Health, noted “this consultation ensures that we develop a framework that not only protects patients but also supports practitioners who are committed to high standards of care. The licensing scheme will be a cornerstone of consumer protection in the aesthetics industry.”
The British Association of Medical Aesthetic Complications (BAMAN) has been talking about the necessity for robust regulations to prevent botched procedures. They see this as key to safeguard patient well-being. Data from Save Face indicates that over 3,000 botched procedures were reported in the UK last year. BAMAN sees this as a clear signal for an urgent regulatory intervention.
The Push for Standardised Training
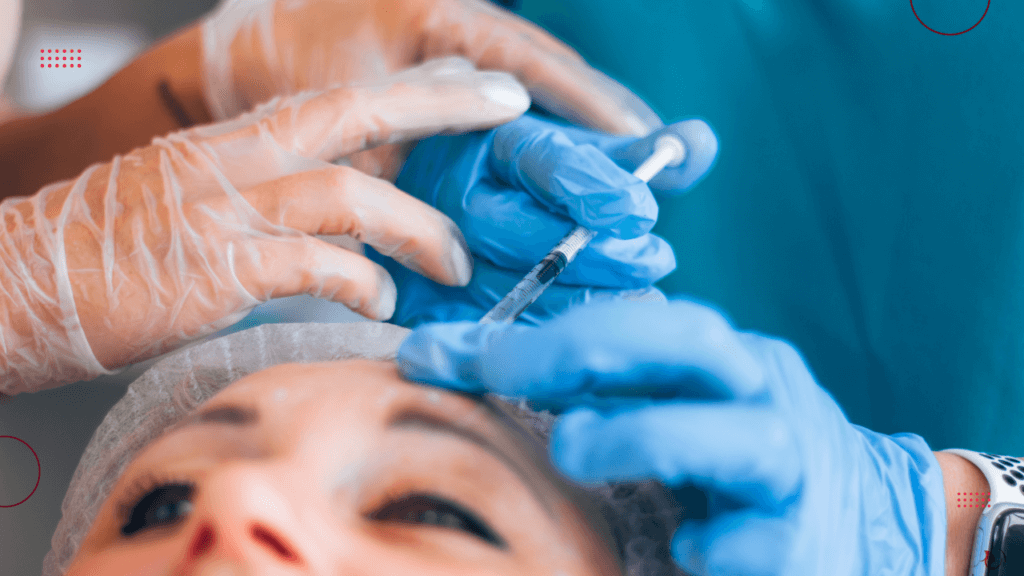
One of the most vocal advocates for reform has been the Joint Council for Cosmetic Practitioners (JCCP). The organisation has long called for standardised education and mandatory licensing. Its proposals include defined competencies for practitioners. Those are ranging from anatomy and physiology to infection control and clinical assessment skills. They have launched a online complaints portal, which allows individuals to report their concerns over aesthetic treatments they have experienced.
They JCCP have also taken a stance against remote prescribing. In a blow to the concept of beauty-related telemedicine, τhe JCCP firmly is opposed to remote prescribing for cosmetic procedures. A policy which is aligning with the Nursing and Midwifery Council’s (NMC) determinations to ensure safe prescribing practices. A lot of the positions of the JCCP have been supported by the British Association of Cosmetic Doctors (BACD).
“Aesthetics is no longer a fringe industry. With its growing popularity comes a responsibility to ensure that practitioners are equipped to deliver safe, effective care. Voluntary systems have proven insufficient; it’s time for robust, enforceable standards.” – Professor David Sines (JCCP Chair)
This shift towards standardisation is expected to reshape the industry. Clinics may face higher costs as they invest in training and compliance. However without these measures safeguarding patients will continue to be a challenge.
In addition to practitioner licensing, there is a growing focus on the regulation of products used in aesthetic treatments. The Medicines and Healthcare Products Regulatory Agency (MHRA) has signalled intentions to introduce stricter rules for the distribution and use of medical devices, such as dermal fillers. You did not read that wrong. Currently dermal fillers are classified as medical devices under UK law. As such, these products are not subject to the rigorous clinical trials required for medicines.
Updates to the Cosmetic Products Enforcement Regulations are also on the horizon. These changes aim to align product quality standards with the proposed licensing scheme, ensuring that both the tools and the hands wielding them meet stringent safety criteria.
| Legal Aspect | Description |
|---|---|
| EU Regulation (Pre-Brexit) | CE Marking |
| UK Regulation (Post-Brexit) | UKCA Marking |
| Device Classification | Most dermal fillers are Class III medical devices |
| Prescription Requirement | Non-prescription |
| Potential Future Regulation | May require prescription or stricter regulation |
| Practitioner Licensing | Movement towards licensing and training requirements |
| Public Safety | Increased focus on public safety due to complications from unqualified practitioners |
| Age Restriction | Botulinum Toxin and Cosmetic Fillers (Children) Act 2021 restricts use for under-18s |
Consumer Safety
The drive for reform has been underscored by a series of high-profile mishaps. In September 2024, Alice Webb, a 33-year-old mother of five, tragically died following a non-surgical BBL procedure in Gloucestershire. Her case sparked widespread outrage and intensified calls for government action. Similarly, Charlotte Booth, 36, suffered permanent disability and nearly lost her life after undergoing a liquid BBL in May 2023.
These incidents are not isolated. Reports of complications from aesthetic procedures have surged. Complaints are ranging from mild dissatisfaction to severe injuries. The British Association of Aesthetic Plastic Surgeons (BAAPS) has been vocal in its criticism. It has been urging the government to adopt “Alice’s Law”. This is a proposed ban on high-risk procedures such as liquid BBLs being performed by unqualified practitioners. Which on balance, seems fair.
Dr. Mary O’Brien, BAAPS President, remarked, “These tragedies are preventable. Procedures like the liquid BBL carry significant risks, including infection, sepsis, and even death. They should only be performed by qualified, registered surgeons who understand the complexities of the human body.”

A similar case of untrained practitioners in South London performing invasive fat removal procedures caused a stir in England. It has led to calls for much stricter regulation in training settings.
The proposed regulatory framework represents a key moment for the aesthetics sector. By raising the bar for entry into the profession and introducing stringent oversight, the reforms could significantly enhance patient safety and elevate industry standards. However, implementation will require careful planning, substantial investment, and ongoing collaboration among regulators, practitioners, and industry stakeholders.
Critics warn that increased regulation could lead to higher treatment costs. This could potentially drive consumers toward unlicensed or overseas providers. Addressing this concern will be critical to the success of the reforms. Meanwhile, industry leaders see an opportunity to professionalise the sector. They are seeking to position it as a respected branch of healthcare rather than merely a subset of the beauty industry.
The Future for Aesthetics
The UK’s aesthetics industry stands at a crossroads. While its growth has brought significant benefits to practitioners and patients, recent mishaps call for urgent reform. The proposed licensing scheme is a step in the right direction. It’s offering a path toward a safer, more professionalised industry. However, its success will depend on effective enforcement and a collective commitment to upholding the highest standards of care.
As public awareness grows and demand for aesthetic treatments continues to rise, ensuring consumer safety must remain at the heart of the industry’s evolution. For Alice Webb and countless others, these reforms cannot come soon enough.



1 Comment
It is about time it was clamped down on we are seeing more people on social media doing a day training to stick needles into other people this should not be happening, beauty industry has gone mad with so called experienced in things they did for a day , health un safety certainly has not gone mad on this industry . There are salons popping up everywhere surly this should be doctored for people safety, it’s just easy to get stuff on the internet world gone mad .